Surviving in extreme environments
Some like it hot
One reason why heat is used in cooking is to kill off all microbes. Book-knowledge used to tell us that life requires water, so there’s no life beyond the boiling point. However, as early as around the middle of the 19th century, the first research about heat-resilient forms of life emerged. Today we know that thermophiles not only survive hot conditions but thrive particularly beyond 50 degrees centigrade (122 degrees Fahrenheit). Organisms whose optimal temperature range is above 70 degrees centigrade (158 degrees Fahrenheit) are called hypothermophiles. The current record holder is Methanopyrus kandleri, an aercheon that was discovered in 2008. It can reproduce at 122 degrees centigrade (252 degrees Fahrenheit) under application of the pressure prevailing in its habitat at an ocean depth of 4,000 meters (13,120 feet), i.e., about 400 bar (5,800 psi).
Archaea such as Methanopyrus kandleri are particularly popular with researchers exploring the origins of life, because these unicellular organisms are found in hot springs, geysers and submarine volcanic pipes, in other words in places where billions of years ago life on Earth more than likely began.
Because many industrial processes are accelerated by high temperatures there is strong interest in making use of the abilities of heat-resilient microorganisms. Or, more precisely, those of their enzymes, i.e., the molecules that activate and sustain metabolic processes. Such thermophilic enzymes enhance detergents or help shift industrial synthesis processes from refineries to bioreactors. Temperature-resistant enzymes can also be of valuable help in separating emulsified greases and oils in sewage. Researchers also see great potential in the area of renewable energies, whether in high-temperature fermentation for biogas production or glucose cracking as a biofuel precursor.
In the fight against the coronavirus pandemic, the thermophilic DNA polymerase enzyme did an important job – as an actor of the polymerase chain reaction, or PCR for short. PCR serves to reproduce the sample material obtained so that a laboratory can work with it. In a high-temperature step at around 95 degrees centigrade (203 degrees Fahrenheit), the DNA double helix of a sample is melted into two strands and, based on two synthetically created counterparts (primers), doubled at temperatures between 45 and 68 degrees centigrade (113 to 154 degrees Fahrenheit). One million copies exist as early as after the 20th PCR cycle, and 100 billion in one afternoon. If this chain reaction does not occur in the case of a covid test, the PCR test is negative.
Prior to the coronavirus pandemic hardly anyone outside of laboratories knew the abbreviation PCR even though biochemist Kary Mullis, who died in 2019, had discovered the polymerase chain reaction as far back as in 1983, which earned him the Nobel Prize in Chemistry ten years later. With good reason because hardly any bioscientific field is conceivable without PCR – from medicine to paleontology to genetic fingerprinting in forensic science.
2,000 years
That’s how long the extremophile plant Welwitschia mirabilis can live. It can do so even in one of the most unhospitable places on our planet, the Namib Desert, with daily temperature fluctuations of more than 50 degrees centigrade (122 degrees Fahrenheit), frequent sand storms and decades-long dry phases. Its survival trick: In addition to a tap root with a length of 15 meters (50 feet), the plant forms a delicate root crown with a diameter of up to 15 meters (50 feet). With it, the plant absorbs night and morning dew. Welwitschia mirabilis is one of the plants that have existed on Earth for the longest time. Scientists assume that it has been around for more than 100 million years.

No fear of freezing
Coldness doesn’t offer as much scope for evolution as high temperatures do. The reason is that at freezing temperatures ice crystals form also in cells and can mechanically destroy them and other structures of living things. That’s why in laboratory settings crystallization is avoided by immersing microorganisms or tissue in liquid nitrogen for flash freezing. The cooling process below the freezing point is so fast that crystals cannot grow large and the frozen water forms a uniform phase.
In nature, two opposite strategies for avoiding frost damage are found. Psychrophiles, i.e., organisms that are optimally adapted to temperatures of 15 degrees centigrade (59 degrees Fahrenheit) and below rely primarily on the use of anti-frost proteins (AFPs). They prevent the formation of ice crystals. AFPs were first discovered in artic fish but are also found in insects, plants, fungi and bacteria.
In some countries, anti-frost proteins from fish or AFPs produced using genetically modified yeast are already being added to food, for instance to make ice cream particularly creamy. Anti-frost proteins could also curb freezer burn of frozen food, i.e., the drying of external layers during thawing processes.
Cryophilic organisms can even withstand –10 degrees centigrade (14 degrees Fahrenheit) and below. Their trick is ice nucleation. During this natural flash freezing process proteins accelerate the formation of ever new ice nuclei (nucleation), thus preventing the cell-destroying growth of crystals. This strategy is found with frost-tolerant insects. Even some frogs can freeze and unfreeze a large part of their water content unharmed in that way.

There are potential biotechnical uses in that area as well. The ice nucleation protein of the Pseudomonas syringae bacterium, for instance, triggers the ice formation of water drops already at –2 degrees centigrade (28 degrees Fahrenheit), which makes it a perfect helper in the production of artificial snow. For comparison, the freezing process of natural snow caused by condensation germs such as mineral dust or soot begins only at –15 degrees centigrade (5 degrees Fahrenheit). On YouTube, impressive videos can be accessed in which the protein causes water in test tubes to freeze within fractions of a second. The problem is that P. syringae can damage plants. Although devitalized bacteria are used for artificial frost acceleration this technology is prohibited in some countries due to its potential damage to nature.
Natural surfactants that form bacteria in extreme cold to dissolve their food as easily digestible droplets have major economic potential as well. Such cold-resistant surfactants, for instance, could be used to prevent thickening of biodiesel at low temperatures. Or to enhance the effectiveness of detergents at lower temperatures. Cold-resilient surfactants could also be used for clean-up purposes in the event of environmental catastrophes in cold regions. Cold-resilient surface coating, of aircraft wings for instance, would be another biotechnical application in this area.
Living in salt
Salts not only flavor our food. As a building block of the body and metabolic players, they’re even of vital importance. But only in small doses. In humans, a mere 0.5 to 1 gram of common salt per kilogram of body weight can lead to death. However, there are countless microorganisms that can live in dry or wet habitats with a high salt content such as the Dead Sea. They’ve developed special protective mechanisms with which they optimize their salt balance. The names of many of these microorganisms contain the stem halo from the Greek word halos for salt, such as the Halomonas bacterium. It has developed ways enabling it to compensate for changing salt concentrations between 0.5 and 25 percent.

A key compound that biotech professionals find particularly exciting protects bacteria such as Halomonas against UV radiation, extreme temperature fluctuation, dehydration and high salt or acid concentrations. It is called ectoine. Ectoine has a hydrating effect and forms a very stable yet air-permeable layer of water around the bacteria – characteristics that the cosmetics industry likes to use. Thanks to its anti-inflammatory and nourishing properties it is added to creams and ointments. The pharmaceutical industry is a fan of ectoine as well. There, it is a component of many eye and nose drops as well as of medicines against respiratory diseases such as asthma.
To access ectoine in industrially usable quantities, the bacteria are “milked.” To do so, they’re initially placed into an environment with an extremely high salt content so that they’ll form lots of ectoine. That is followed by a bath in a salt-free solution. To survive there, Halomonas has to rid itself of the excess ectoine again and releases it into the solution from which it can be extracted.
The bacteriorhodopsin membrane protein from the extremely salt-resistant Halobacterium salinarum on the other hand fascinates many scientists because of its special abilities to react to light. Since as far back as the end of the 1990s, bacteriorhodopsin has been in biotechnical use, as a security pigment and as the first biomolecular data storage medium, among other things. In addition, the discovery of the bacteriorhodopsin of Halobacterium salinarum has opened the young research field of optogenetics. The bacterial building block resembles a rhodopsin in the human eye. Researchers therefore think it’s possible that this similarity may open pathways to curing neural disorders.
1.2 millimeters (0.05 inches)
is the maximum size of tardigrades (aka water bears). Aside from that, tardigrades are pretty – spectacular. They’re deemed to be the most extremophile animal with good reason. 100 degrees centigrade (212 degrees Fahrenheit) or -273 degrees centigrade (-459.4 degrees Fahrenheit) – no problem. Even vacuums and cosmic radiation cannot harm this resilient half-pint as tests in space have shown. A decade without food and water – easy. After the first drop, the water bear wakes up as if nothing had happened, metabolizes and procreates. Its extreme resilience is based on sacrifice: tardigrades have no heart, breathe through their skin and use individual cells that can distinguish between bright and dark as their eyes.

Acidity boosts ingenuity
We tend not to spend a lot of time thinking about the acid-base equilibrium of our environment except perhaps when choosing plants for our gardens. The normal pH in most biological contexts is 7.0 to 7.2. However, acidophile microbes are also found in extremely acidic milieus down to pH 0, and their alkaliphile counterparts in extremely alkaline environments of up to around pH 12.
Acidophiles often appear in context with volcanic activity, for instance near volcanic springs emitting hydrogen sulfide, among other things. As a result, acidophiles are usually extremely thermophile as well. They’ve already been in use for centuries in mining, in so-called bioleaching. In bioleaching, low-concentration ores are heaped and irrigated, which arouses the microbes that already exist within the ores. They attack the rock and release the desired metal that can ultimately be extracted from the draining slurry.
The underlying microbiological processes remained obscure for a long time. It was not until the late 20th century that the operation of the acidophiles began to be understood and known strains were systematically used. Today, for instance, microbes make extremely hard-to-access gold resources accessible in industrial tanks. In uranium mining, the injection of suitable culture media into underground deposits is common practice.
Alkaliphiles can be used industrially as well. The enzymes of alkaliphile bacteria are of particular interest to detergent manufacturers. The amylase enzyme decomposes carbohydrates, protease deals with proteins and lipase cracks greases. Heat reinforces their effects – and that’s where things become complicated. Unlike many acidophiles, alkaliphiles are not very heat-resistant. Exactly that is where researchers took action and developed the Thermopallium natronophilum bacterium in the genetic laboratory. Its enzymes are also active at high washing temperatures up to the limit of 100 degrees centigrade (212 degrees Fahrenheit).
The Conan bacterium
Arnold Schwarzenegger became world-famous due to his role as the hulky and invincible Conan the Barbarian. That Deinococcus radiodurans is referred to as the Conan among bacteria says a lot about its resilience. Some representatives of D. radiodurans survived a radiation dose of 17,500 Gy. For comparison, humans die within one to two weeks following exposure to a radiation dose of 7 to 10 Gy. In addition, “Conan,” as a polyextremophile organism, is resistant against dehydration and enjoys very good protection against UV rays. A working group at the University of Vienna has already tested these bacteria outside the International Space Station (ISS) for their resilience in space – the microbes survived a stay there for three years. Astrobiologists therefore consider it possible that life can spread from one planet to another through the transportation of germs. In that way, some of them might have found their way to Earth about 3.5 trillion years ago and formed the origin of life on this planet.
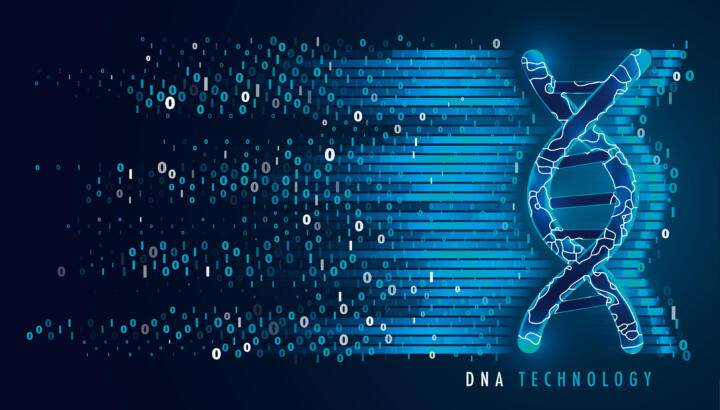
Due to their resilience, Deinococcus radiodurans could also be of interest as a highly resilient data storage medium. The idea is for data to be stored in the bacteria in the form of artificial DNA. Initial successes have already been achieved. High storage density is one of the advantages of artificial DNA as a data storage medium. According to more recent research from the United States, one billion gigabytes of data can be stored on one cubic millimeter (6.10237e-5 cubic inches) of DNA. The entire internet could be stored in a medium the size of a shoe box and infinitely replicated by means of DNA sequencing.
Don’t crack under pressure
Under the ocean surface, the hydrostatic pressure increases for every ten meters (33 feet) of depth by one atmosphere (approximately one bar / 14.5 psi). Consequently, in the deepest depressions of the Pacific (Challenger Deep: 10.929 m / 35,586.3 ft) pressures of more than one kilobar (0.1 GPa), equating to the weight of one metric ton (1.1 short tons) per square centimeter (0.001 square feet) prevail. Yet even there, living organisms known as barophiles, which have adapted to those conditions that would be fatal for us surface inhabitants, are found. For comparison, scuba divers using standard equipment reach depths of down to 60 meters (197 feet).
Many deep sea creatures are found in the vicinity of hot springs (pictured) and are adapted to high temperatures accordingly. The energy supply in the deep sea is based on chemosynthesis – such as oxidation (“combustion”) of reduced metals that escape from hydrothermal springs. However, a diversity of species that as yet has been largely unexplored is also found in the vicinity of manganese nodules that are lying around in some places of the sea bottom and planned to be mined commercially in the near future.

Contrary to expectations, living things that can cope with conditions far away from sunlight and under high pressure are found even in deep rock strata. Both the bottom of the deep sea and the deeper substratum are some of the least explored parts of the Earth’s biosphere. The large-scale Deep Carbon Observatory (2009–2019) project that was run for a period of ten years came to the conclusion that at least two billion cubic kilometers (0.47 billion cubic miles) of biotopes are located underneath our feet – equating to more than twice the volume of all oceans combined. The extrapolated biomass of the underground rock dwellers amounts to around 20 billion metric tons (22 billion short tons), a multiple of the total mass of the human population on Earth.
Some of these microbes are of industrial interest because they’re involved in the production of oil and gas deposits. Others assist in mining uranium ores.
Subterranean life is particularly exciting for astrobiology as well because comparable biotopes definitely exist on Mars too.
Desulforudis audaxviator, a species initially found in South Africa at a depth of 2.8 kilometers (1.73 miles), where it appeared to be the only microbial species in its habitat, caused a sensation. Does it really operate an ecosystem all by itself? Or might other species be so firmly anchored in the rock that they couldn’t be extracted? Further surprises followed when D. audaxviator was found on other continents. How the species that is killed off by atmospheric oxygen managed its long-distance travel remains to be resolved.



